Project B-T1 - Cerebellar control of eye motion dynamics: from animal model to patients
Functional organization and evolutionary conservation of eye movements
Spatio-temporally appropriate eye movements are indispensible for accurate retinal image processing. Different types of eye movements with specific dynamic characteristics cover the functional range to meet the respective visual requirements that allow stabilization of retinal images during locomotor activity (vestibulo-ocular reflex, VOR; optokinetic reflexes, OKR), as well as a shift of visual attention to particular targets in space [1]. Saccades allow changing the fixation of selected targets of interest, while smooth pursuit eye motion enable tracking of small moving visual stimuli. Reflexive eye movements such as the VOR and OKR are mediated by evolutionary conserved brainstem pathways that ensure the sensory-motor transformation of visuo-vestibular signals [1]. The remarkable conservation of the morpho-physiological substrate suggests the existence of basic neural circuits with cellular mechanisms that are independent of ocular motor range or general performance as evidenced by the presence of almost identical functional components from fish to mammals. Accordingly, more simple animal models such as amphibians are ideally suited to decipher general principles at the cellular and circuit level that allow understanding of how precise eye movements are achieved.
Role of the cerebellum in eye movement control
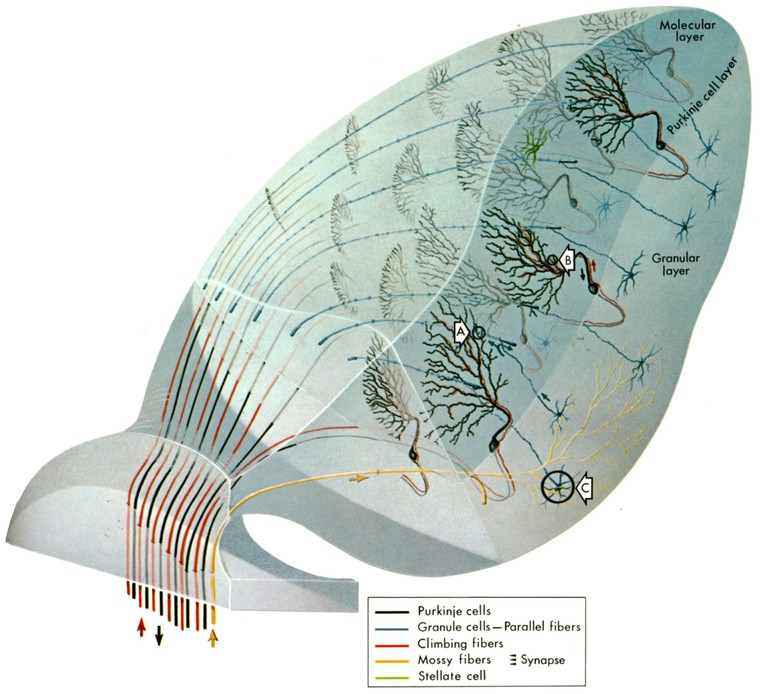
The cerebellum as an evolutionarily rather conserved structure (Fig. 1) plays an essential role for the control of eye movements. Different areas of the cerebellum, such as the vermis, fastigial nuclei or the floccular lobes are responsible for the precise execution of specific types of eye movements [2]. The most conserved part - the vestibulo-cerebellum - is intricately linked with the vestibular system (Fig. 2) and controls different spatio-temporal aspects of the VOR and its adaptation during visuo-vestibular conflict. Such a sensory discrepancy can be experimentally induced to provoke motor learning and excert short- and long-tem plasticity. The most commonly encountered eye movement deficits following cerebellar damage include saccadic hypo- or hypermetria and a pathological nystagmus that affects the normal execution of both OKR and VOR. Pharmacological treatment of eye movement deficits caused by cerebellar damage is difficult, but has been improved recently by a new treatment with 4-aminopyridine (4-AP) [3], a highly potent potassium channel blocker that acts specifically on cerebellar Purkinje cells [4]. This link between a particular ion channel and ocular motor performance offers the possibility to understand how the cerebellar/brainstem circuitry achieves the spatio-temporal precision and plasticity of eye movements.
Objectives and description of the project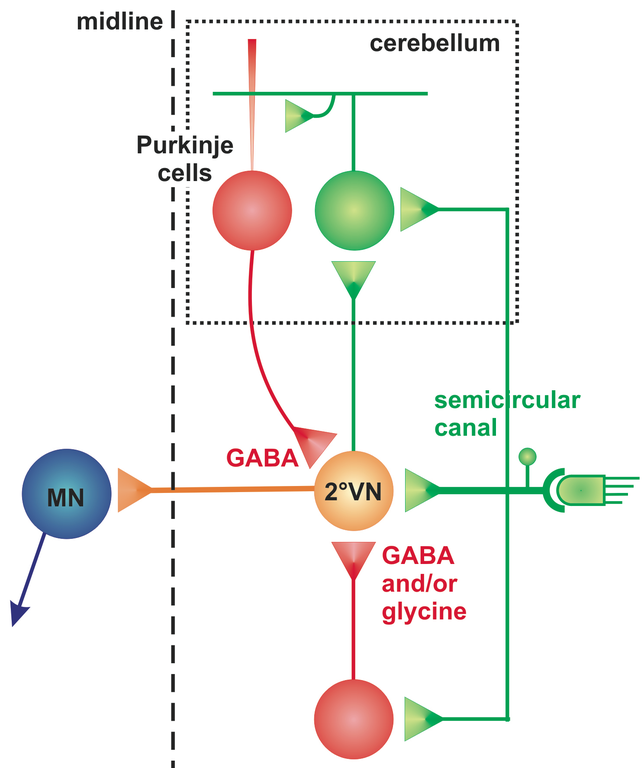
The current project aims to elucidate in a multidisciplinary and translational approach the functional role of cerebellar circuits in generating precise metrics, dynamics and plastic adaptations of eye movements. Empiric experiments in amphibians and humans are combined with computational implementation of neural signal processing in a multi-level mathematical model. The unique accessibility of semi-intact Xenopus in vitro preparations with intact sensory-motor structures underlying the VOR for detailed pharmaco-physiological manipulations offers the possibility to reveal the cellular mechanisms by which the cerebellum influences brainstem ocular motor control centers (Straka lab). Eye movement recordings in control subjects and cerebellar patients allow studying the functional consequences of 4-AP administration on the spatio-temporal precision of reflexive and voluntary eye movements (Kutz and Strupp labs). Computational modeling will integrate the results of both empiric approaches to simulate cerebellar signal transformation underlying the generation of precise eye movements (Glasauer lab).
References
[1] Straka H, Dieringer N (2004) Basic organization principles of the VOR: lessons from frogs. Prog Neurobiol 73: 259-309.
[2] Glasauer S (2003) Cerebellar contribution to saccades and gaze holding: a modelling approach. Ann NY Acad Sci 1004: 206-219.
[3] Kalla R, Glasauer S, Büttner U, Brandt T, Strupp M (2007) 4-Aminopyridine restores vertical and horizontal neural integrator function in downbeat nystagmus. Brain 130: 2441-2451.
[4] Alvina K, Khodakhah K (2010) The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci 30: 7258–7268.